Project 15. Investigating the life cycle of RAD51AP1-mediated R-loops.
Telomeres, initially thought to be transcriptionally inactive, are transcribed and produce non-coding RNA known as TERRA (telomeric repeat-containing RNA). TERRA RNA can hybridize with telomeric DNA to form telomeric R-loops (tR-loops). These tR-loops are elevated in ALT-positive cancers and have been directly implicated in the regulation of ALT. Specifically, optimal levels of tR-loops are required to induce constitutive replication stress for recombination to occur between telomeres via ALT. Too many or too few tR-loops are detrimental to the survival of ALT cancer cells.
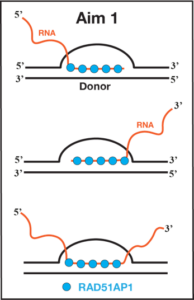
RAD51-associated protein 1 (RAD51AP1) is a pro-HR factor that functions with RAD51 recombinase to promote D-loop formation during HR. Cells lacking RAD51AP1 exhibit hypersensitivity to DSB-causing genotoxic agents, and RAD51AP1 is found to be upregulated in various cancers. In recent years, RAD51AP1 has emerged as a necessary ALT factor, primarily due to its capacity to form R-loops via its RNA-strand invasion activity into dsDNA. RAD51AP1 contributes to ALT at two levels. First, RAD51AP1 forms telomeric R-loops using TERRA to maintain a critical level of replication stress to sustain an optimal level of ALT. Second, RAD51AP1-mediated R-loops aid in proficient D-loop formation by transiently opening the dsDNA duplex to facilitate efficient DNA strand invasion. Similarly, during HR, when DSBs occur in actively transcribed genes, the RNA transcripts are used by RAD51AP1 to make R-loops in the donor duplex to promote efficient D-loop formation. The transitory DNA structure, where both R- and D-loops exist in the same duplex, is known as DR-loops. In light of these recent findings, we have only begun to appreciate the multifaceted roles of RAD51AP1 in DSB repair and ALT. Many outstanding questions regarding RAD51AP1’s function in DSB repair and ALT remain unanswered.
In this project, we will primarily investigate RAD51AP1’s R-loop formation activity using in vitro biochemistry and determine the mechanistic basis of RAD51AP1 functions in DSB repair and ALT. Specifically, we will elucidate the mechanisms of genomic and telomeric R-loop formation by RAD51AP1, the transition of R-loops to D-loops (DR-loop switch), and DNA synthesis post-DR-loop switch. We will tackle the following questions to discover RAD51AP1’s function in maintaining genomic stability and ALT.
-
How does RAD51AP1 form genomic and telomeric R-loops?
-
What is the mechanism of the R-to-D loop switch?
-
How does DNA synthesis initiate from DR-loops?
To achieve these aims, we will use bulk biochemistry and single-molecule imaging methods. We will form R-loops (both genomic and tR-loops) outside the cells using synthetic DNA and purified protein(s). To gain unprecedented details of R-loop formation by RAD51AP1, we will study these reactions at the single-molecule level using optical tweezers with confocal microscopy (C-trap, Lumicks). In summary, this project will uncover the molecular mechanisms of the life cycle of R-loops to deepen our understanding of DSB repair and ALT, illuminating key aspects of cancer development and survival.
