Project 1. R-loops modulating telomere maintenance and life span in fission yeast.
Telomeres are the nucleoprotein complexes that protect the ends of linear chromosomes from degradation and fusions. Telomeric DNA is transcribed into telomeric RNA (TERRA), which forms R-loops near chromosome ends. It has recently become clear that R-loops play a critical role in telomere maintenance and thereby in regulating the rate of replicative senescence. When telomeres in fission yeast lacking telomerase reach a critical length, cells enter a state of crisis in which chromosome ends are no longer distinguished from DNA double-strand breaks and processing by DNA repair factors commences. Some cells escape this crisis by activating alternative pathways of telomere maintenance based on homologous recombination (HR). Here, short telomeres repeatedly invade and copy homologous sequences from other chromosomes to replenish terminal sequences. Such HR-based telomere lengthening has been observed in diverse species including yeasts and human cells.
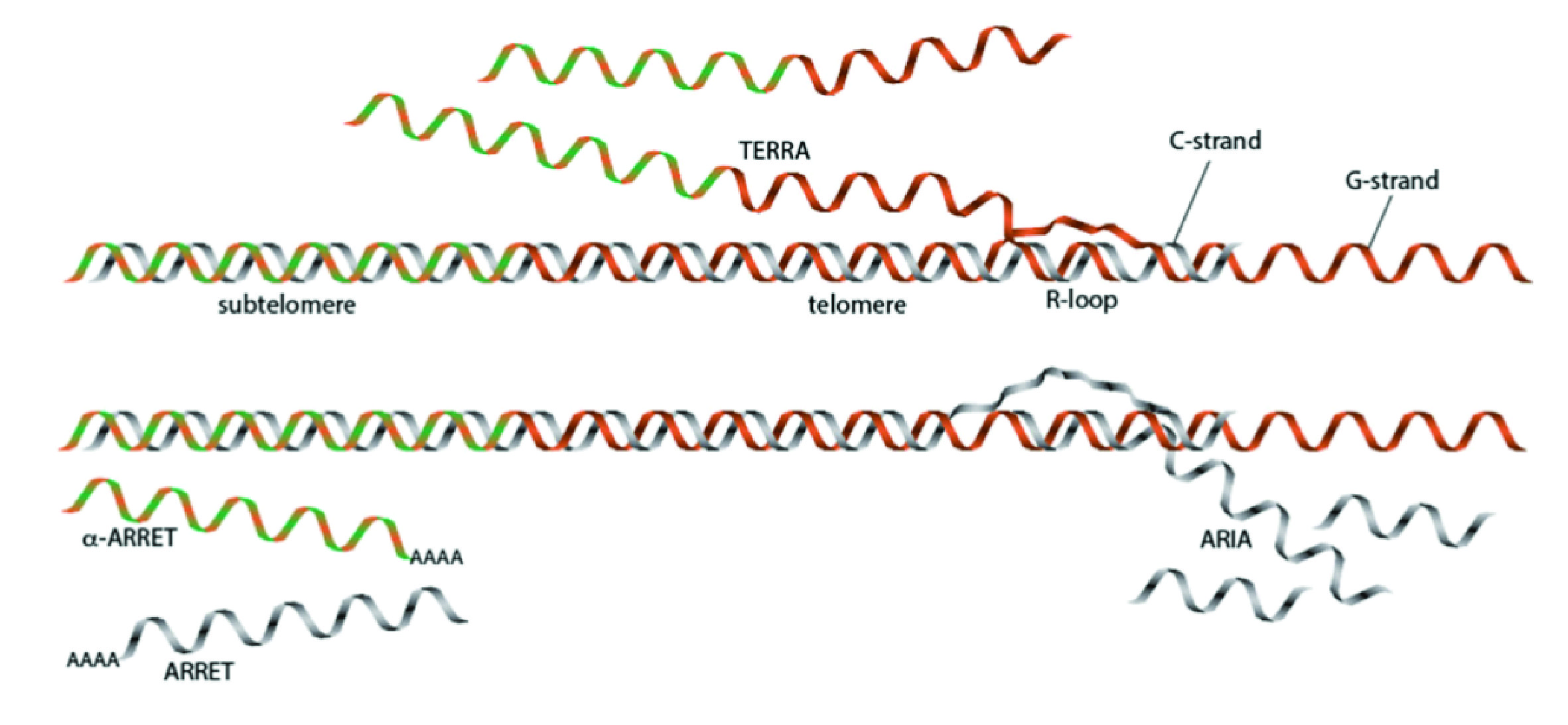
As part of this RTG, a graduate student working in the Baumann group will now define the roles of R-loops in chromosome end maintenance in the presence of functional telomerase. In fission yeast, four groups of telomeric transcripts have been described (Figure 1). By selectively manipulating their expression level, their retention at the site of transcription and their stability, we will investigate how the different RNA-DNA hybrids affect telomere replication and telomerase activity. In the context of telomerase function, we are particularly interested in determining whether the ARIA transcripts function as a competitive inhibitor of telomerase recruitment, as ARIA can form RNA-DNA hybrid structures with the single-stranded overhangs at chromosome ends. These are the very same DNA regions that form the RNA-DNA hybrid structure with the alignment region of the telomerase RNA subunit to permit reverse transcription of the template into telomeric DNA repeats. As part of our characterization of telomerase biogenesis and function, we have generated several tools that we will now employ to assess the role of telomeric RNA in the recruitment and regulation of telomerase to chromosome ends. In addition to the focus on telomerase, this project will also investigate the effects that the various telomeric RNA-DNA hybrid structures have on lagging strand DNA synthesis and on the rate of telomere resection between cycles of telomerase engagement. Here we will examine the hypothesis that R-loops play a role in regulating telomere recombination even when telomerase is present in cells.
